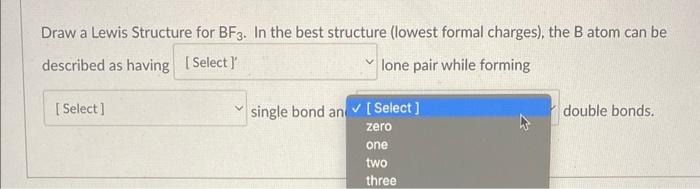
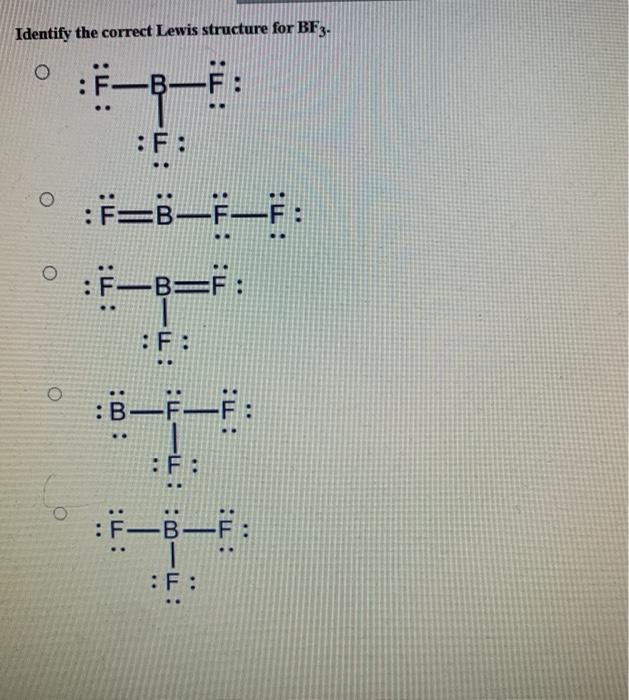
3. ) Boron trifluoride contains one boron atom and three fluorine atoms. Lewis structure of boron trifluoride (BF 3) is shown below and you can see each fluorine atom has made a single bond with boron atom. Boron atom is the center atom. In this tutorial, we will learn how to draw the lewis structure of BF 3 with all theories.
Solved Identify the correct Lewis structure for BF3. Ë–F .. | Chegg.com
Jun 21, 2023Steps of drawing BF3 lewis structure Step 1: Find the total valence electrons in BF3 molecule. In order to find the total valence electrons in BF3 molecule, first of all you should know the valence electrons present in boron atom as well as fluorine atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.). Here, I’ll tell you how you can easily find
Source Image: chegg.com
Download Image
Contents Molecular Geometry of BF3 BF3 Lewis Structure BF3 Hybridization BF3 Polarity Molecular Geometry of BF3 The geometry of molecule of BF3 is ‘Trigonal Planar.’ With the reference of Chemistry, ‘Trigonal Planar’ is a model with three atoms around one atom in the middle.

Source Image: homework.study.com
Download Image
BF3 Lewis Structure: How to Draw the Lewis Structure for BF3 – YouTube The first step is to sketch the Lewis structure of the BF3 molecule, to add valence electrons around the boron atom; the second step is to add valence electrons to the three fluorine atoms, and the final step is to combine the step1 and step2 to get the BF3 Lewis Structure.
Source Image: chegg.com
Download Image
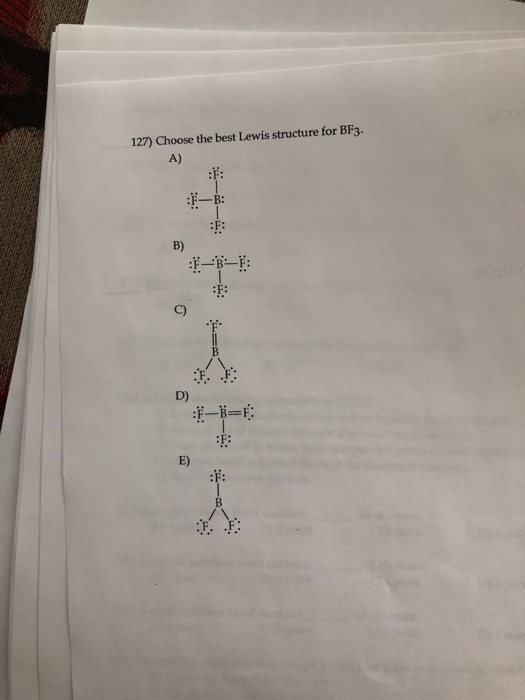
Choose The Best Lewis Structure For Bf3
The first step is to sketch the Lewis structure of the BF3 molecule, to add valence electrons around the boron atom; the second step is to add valence electrons to the three fluorine atoms, and the final step is to combine the step1 and step2 to get the BF3 Lewis Structure. Chemistry learning made easy.This tutorial will help you deal with the lewis structure and moleculargeometry for boron trifluoride (BF3).
Solved 127) Choose the best Lewis structure for BF3. A) B) | Chegg.com
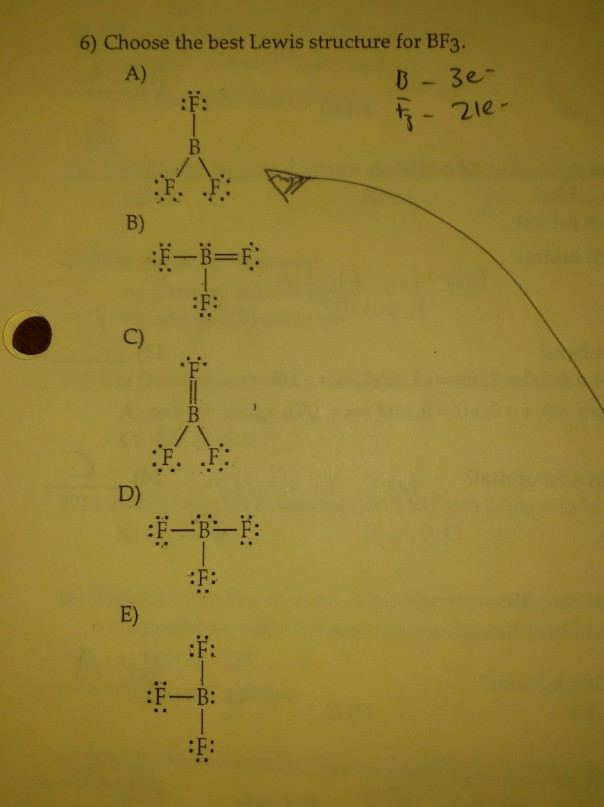
May 22, 2023Watch on 5 Steps to Draw the Lewis Structure of BF3 Step #1: Calculate the total number of valence electrons Here, the given molecule is BF3. In order to draw the lewis structure of BF3, first of all you have to find the total number of valence electrons present in the BF3 molecule. Solved 6) Choose the best Lewis structure for BF3. A) B-3e- | Chegg.com
Source Image: chegg.com
Download Image
BF3 Lewis Structure (Boron Trifluoride) May 22, 2023Watch on 5 Steps to Draw the Lewis Structure of BF3 Step #1: Calculate the total number of valence electrons Here, the given molecule is BF3. In order to draw the lewis structure of BF3, first of all you have to find the total number of valence electrons present in the BF3 molecule.

Source Image: pinterest.com
Download Image
Solved Identify the correct Lewis structure for BF3. Ë–F .. | Chegg.com 3. ) Boron trifluoride contains one boron atom and three fluorine atoms. Lewis structure of boron trifluoride (BF 3) is shown below and you can see each fluorine atom has made a single bond with boron atom. Boron atom is the center atom. In this tutorial, we will learn how to draw the lewis structure of BF 3 with all theories.
Source Image: chegg.com
Download Image
BF3 Lewis Structure: How to Draw the Lewis Structure for BF3 – YouTube Contents Molecular Geometry of BF3 BF3 Lewis Structure BF3 Hybridization BF3 Polarity Molecular Geometry of BF3 The geometry of molecule of BF3 is ‘Trigonal Planar.’ With the reference of Chemistry, ‘Trigonal Planar’ is a model with three atoms around one atom in the middle.

Source Image: youtube.com
Download Image
BF3 Lewis Structure, Molecular Geometry, and Hybridization – Techiescientist Let’s explore the Lewis structure of boron trifluoride, BF3. The BF3 molecule comprises a central boron (B) atom and three surrounding fluorine (F) atoms. Boron resides in group 13 of the periodic table, boasting three valence electrons, while each fluorine atom originates from group 17, contributing seven valence electrons. Step-by-Step Guide to Drawing the Lewis

Source Image: techiescientist.com
Download Image
Boron Trifluoride | BF3 Lewis Structure, Properties & Molar Mass | Study.com The first step is to sketch the Lewis structure of the BF3 molecule, to add valence electrons around the boron atom; the second step is to add valence electrons to the three fluorine atoms, and the final step is to combine the step1 and step2 to get the BF3 Lewis Structure.

Source Image: study.com
Download Image
24. Lewis Dot Structure of BF3| How to Draw Lewis Structures| Class 11 Chemistry| Chemical Bonding – YouTube Chemistry learning made easy.This tutorial will help you deal with the lewis structure and moleculargeometry for boron trifluoride (BF3).

Source Image: youtube.com
Download Image
BF3 Lewis Structure (Boron Trifluoride)
24. Lewis Dot Structure of BF3| How to Draw Lewis Structures| Class 11 Chemistry| Chemical Bonding – YouTube Jun 21, 2023Steps of drawing BF3 lewis structure Step 1: Find the total valence electrons in BF3 molecule. In order to find the total valence electrons in BF3 molecule, first of all you should know the valence electrons present in boron atom as well as fluorine atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.). Here, I’ll tell you how you can easily find
BF3 Lewis Structure: How to Draw the Lewis Structure for BF3 – YouTube Boron Trifluoride | BF3 Lewis Structure, Properties & Molar Mass | Study.com Let’s explore the Lewis structure of boron trifluoride, BF3. The BF3 molecule comprises a central boron (B) atom and three surrounding fluorine (F) atoms. Boron resides in group 13 of the periodic table, boasting three valence electrons, while each fluorine atom originates from group 17, contributing seven valence electrons. Step-by-Step Guide to Drawing the Lewis